where
The entropy per mole of an n-component ideal mixture is given by
![]()
where ![]() is the mole fraction of the ith constituent.
If there are only two constituents, we have
is the mole fraction of the ith constituent.
If there are only two constituents, we have
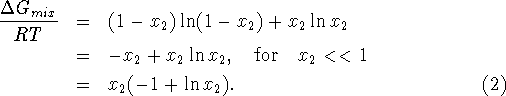
If the mixture has ![]() moles of the second constituent, we get
moles of the second constituent, we get
![]()
The energy required to separate the mixture is
For those who didn't follow the derivation, of (4), we summarize. R is the universal gas constant 8.314 joules per mole per degree Kelvin, T is the absolute temperature at which the separation takes place and x is the mole fraction in the mixture of the substance being extracted.